
Body cells are constantly breaking down carbohydrates into sugar, which is their main source of energy. The human body also uses chemical energy, constantly breaking down the bonds between the atoms that make up food. Chemical energy is also being used when wood burns in a fireplace. This heat is used directly for space heating and industrial processes, converted into motion by vehicles, or converted into electricity by power plant turbines. Fossil fuels are an everyday example: oil, gas and coal are burned to release their chemical energy in the form of heat. When the chemical bonds between atoms are broken, the energy contained is released. Covalent, the type of bond between nonmetal atoms.Metallic, the type of bond between metal atoms.Ionic, the type of bond that forms between metal and nonmetal atoms.The atoms that make up substances are held together by chemical bonds, which contain energy. Here we will provide a simple definition of each energy form, while discussing their most common real-world applications. The US Energy Information Administration provides a classification of the main types of energy: Forms of Potential Energy This can refer to the motion of visible objects and substances, but also the motion of invisible waves and microscopic particles like atoms and molecules. Kinetic energy can be described as “energy in motion”.A compressed spring, an elevated water tank and a charged battery all contain potential energy. Potential energy can be described as “stored energy”.However, all forms of energy can be classified into two broad categories: potential and kinetic. Your stove converts chemical energy from natural gas into heat energy for cooking, and a solar panel converts radiant energy from the sun into electricity. There are many types of energy, and it can be converted from one type to another.
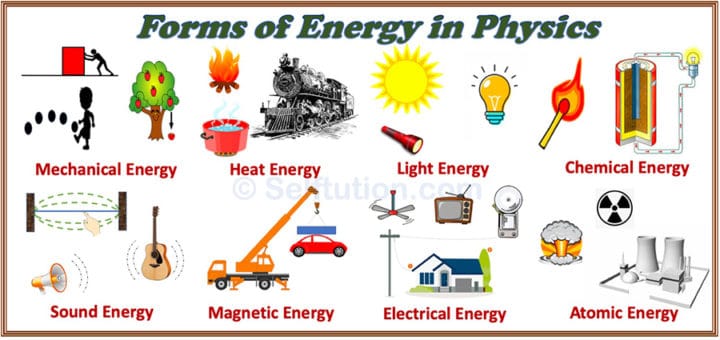
The heat energy released by a furnace or removed by an air conditioner is measured in British Thermal Units (1 BTU = 1,055 Joules).



 0 kommentar(er)
0 kommentar(er)
